Showing 32 compounds.

Catalog Number: SV-Lip-d5-I
Chemical Name: Alpha Lipoic acid-d5-racemic
Type: deuterium labelled drug standard

Catalog Number: SV-Lip-d5-II
Chemical Name: Alpha Lipoic acid-d5-racemic
Type: deuterium labelled drug standard

Catalog Number: SV-Beh-d3
Chemical Name: Betahistine-d3
Type: deuterium labelled drug standard

Catalog Number: SV-Car-d3
Chemical Name: Carisoprodol-d3
Type: deuterium labelled drug standard

Catalog Number: SV-Rot-d7
Chemical Name: Rotigotine-d7
Type: deuterium labelled drug standard

Catalog Number: SV-Dar-d4
Chemical Name: Darifenacin-d4-HBr
Type: deuterium labelled drug standard

Catalog Number: SV-Tiz-d4
Chemical Name: Tizanidine-d4-HCl
Type: deuterium labelled drug standard

Catalog Number: SV-Aba-d4
Chemical Name: Abacavir-d4
Type: deuterium labelled drug standard
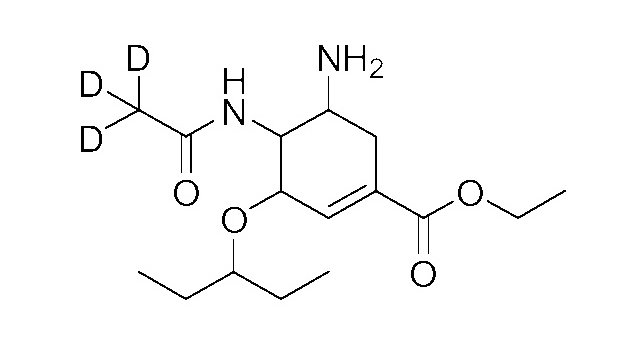
Catalog Number: SV-Ose-d3
Chemical Name: Oseltamivir acid-d3
Type: deuterium labelled drug standard

Catalog Number: SV-Itr-d8
Chemical Name: Hydroxy Itraconazole-d8
Type: deuterium labelled drug standard

Catalog Number: SV-Dan-d4
Chemical Name: Dantrolene-d4
Type: deuterium labelled drug standard

Catalog Number: SV-Dhy-d5
Chemical Name: Diphenhydramine-d5 HCl
Type: deuterium labelled drug standard

Catalog Number: SV-Bps-d8
Chemical Name: Bis-(4-hydroxyphenyl) sulfone-d8
Type: deuterium labelled drug standard

Catalog Number: SV-Mpb-d4
Chemical Name: Methyl paraben-d4
Type: deuterium labelled drug standard

Catalog Number: SV-Dex-d3
Chemical Name: Dexketoprofen-d3
Type: deuterium labelled drug standard

Catalog Number: SV-pip-d3
Chemical Name: Piperaquine-d3
Type: deuterium labelled drug standard

Catalog Number: SV-Met-d3
Chemical Name: Metoclopromide-d3
Type: deuterium labelled drug standard

Catalog Number: SV-Mar-d6
Chemical Name: Maraviroc-d6
Type: deuterium labelled drug standard
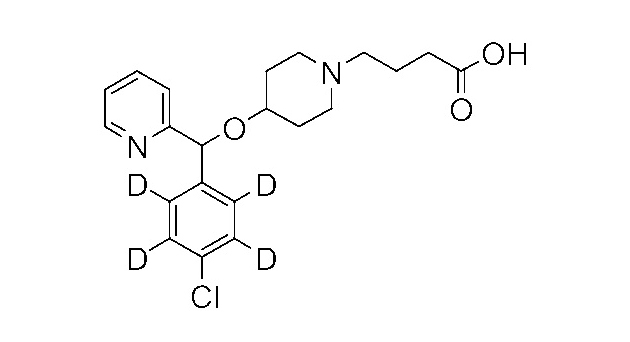
Catalog Number: SV-Bpo-d4
Chemical Name: Bepotastine-d4
Type: deuterium labelled drug standard

Catalog Number: SV-Alv-d5
Chemical Name: Alvimopan-d5
Type: deuterium labelled drug standard

Catalog Number: SV-Iva-d4
Chemical Name: Ivacaftor-d4
Type: deuterium labelled drug standard

Catalog Number: SV-Mir-d5
Chemical Name: Mirabegron-d5
Type: deuterium labelled drug standard

Catalog Number: SV-Cri-d5
Chemical Name: Crizotinib-d5
Type: deuterium labelled drug standard

Catalog Number: SV-Car-d6
Chemical Name: Carbinoxamine-d6
Type: deuterium labelled drug standard

Catalog Number: SV-Amb-d3
Chemical Name: Ambrisentan-d3
Type: deuterium labelled drug standard

Catalog Number: SV-Baz-d4
Chemical Name: Bazedoxifene-d4-acetate
Type: deuterium labelled drug standard

Catalog Number: SV-Ben-d3
Chemical Name: Bendamustine-d3
Type: deuterium labelled drug standard

Catalog Number: SV-Bim-d5
Chemical Name: Bimatoprost-d5
Type: deuterium labelled drug standard

Catalog Number: SV-Bro-d4
Chemical Name: Bromfenoc-d4
Type: deuterium labelled drug standard

Catalog Number: SV-Ret-d4
Chemical Name: Retigabine-d4
Type: deuterium labelled drug standard

Catalog Number: SV-Cel-d7
Chemical Name: Celecoxib-d7
Type: deuterium labelled drug standard

Catalog Number: SV-Ver-d6
Chemical Name: Veratric acid-d6
Type: deuterium labelled drug standard